Abstract:
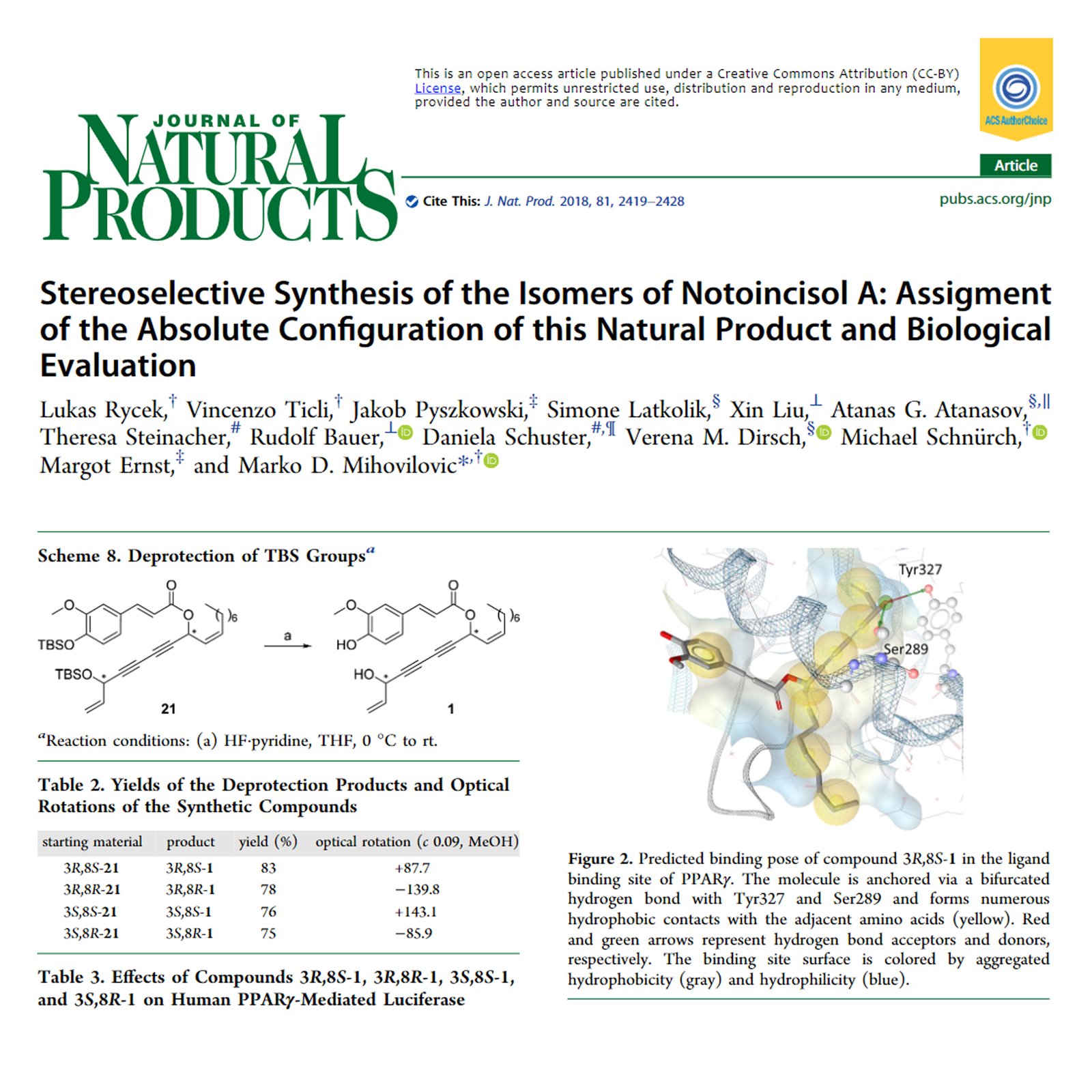
The total syntheses of all stereoisomers of notoincisol A, a recently isolated natural product with potential anti-inflammatory activity, are reported. The asymmetric synthesis was conducted employing a lipase-mediated kinetic resolution, which enables easy access to all required chiral building blocks with the aim of establishing the absolute configuration of the naturally occurring isomer. This was achieved by comparison of optical properties of the isolated compound with the synthetic derivatives obtained. Moreover, an assessment of the biological activity on PPARγ (peroxisome proliferator-activated receptor gamma) as a prominent receptor related to inflammation is reported. Only the natural isomer was found to activate the PPARγ receptor, and this phenomenon could be explained based on molecular docking studies. In addition, the pharmacological profiles of the isomers were determined using the GABAA (gamma-aminobutyric acid A) ion channel receptor as a representative target for allosteric modulation related to diverse CNS activities. These compounds were found to be weak allosteric modulators of the α1β3 and α1β2γ2 receptor subtypes.
Reference:
Rycek L, Ticli V, Pyszkowski J, Latkolik S, Liu X, Atanasov AG, Steinacher T, Bauer R, Schuster D, Dirsch VM, Schnürch M, Ernst M, Mihovilovic MD. Stereoselective Synthesis of the Isomers of Notoincisol A: Assigment of the Absolute Configuration of this Natural Product and Biological Evaluation. J Nat Prod. 2018 Nov 26;81(11):2419-2428. doi: 10.1021/acs.jnatprod.8b00439. Epub 2018, Oct 26. PubMed PMID: 30362739; PubMed Central PMCID: PMC6256351.
Keywords: stereoselective synthesis of the isomers of Notoincisol A, assigment of absolute configuration, natural products, biological evaluation, anti-inflammatory activity, asymmetric synthesis, lipase-mediated kinetic resolution, PPARγ (peroxisome proliferator-activated receptor gamma), molecular docking studies, GABAA (gamma-aminobutyric acid A) ion channel receptor, α1β3 and α1β2γ2 receptor subtypes.
Join for free INPST as a member
The International Natural Product Sciences Taskforce (INPST) maintains up-to-date lists with conferences, grants and funding opportunities, jobs and open positions, and journal special issues with relevance for the area of phytochemistry and food chemistry, pharmacology, pharmacognosy research, and natural product science.