Abstract:
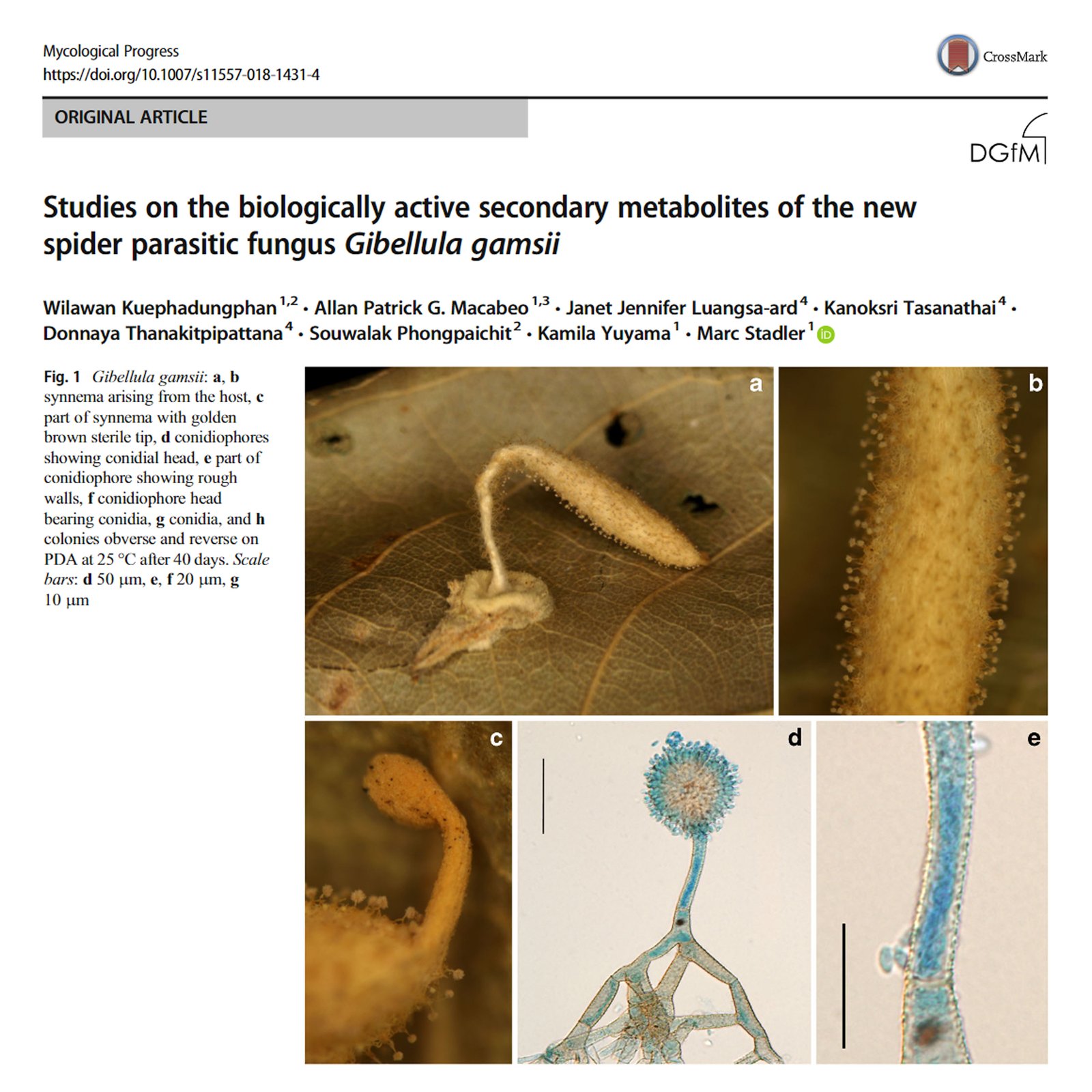
Numerous gatherings of a new species of the genus Gibellula, closely resembling the monotypic, neotropical G. mirabilis were encountered in Thailand. The taxon was cultured successfully although no in vitro sporulation was observed. The new species, Gibellula gamsii, could be distinguished from closely related other Gibellula species on the basis of morphological features and phylogenetic inferences recruiting concatenated sequences of five DNA loci including ITS, LSU, RPB1, RPB2, and EF1-α. The secondary metabolites of G. gamsii, strain BCC47868, were studied concurrently after preparative separation of the crude extract by preparative high-performance liquid chromatography (HPLC). Two new 1,3-disubstituted β-carboline alkaloids, for which we propose the trivial names, gibellamines A (1) and B (2), were isolated. The chemical structures of these compounds were elucidated by interpretation of spectral data, generated by nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS). The alkaloid 1 also exhibited moderate anti-biofilm activity against Staphylococcus aureus.
Reference:
Kuephadungphan, W., Macabeo, A.P.G., Luangsa-ard, J.J. et al. Mycol Progress (2018). https://doi.org/10.1007/s11557-018-1431-4
Keywords: studies on biologically active secondary metabolites, spider parasitic fungus, Gibellula gamsii, anti-biofilm activity against Staphylococcus aureus, gibellamines A and B, 1,3-disubstituted β-carboline alkaloids, neotropical G. mirabilis.
Join for free INPST as a member
The International Natural Product Sciences Taskforce (INPST) maintains up-to-date lists with conferences, grants and funding opportunities, jobs and open positions, and journal special issues with relevance for the area of phytochemistry and food chemistry, pharmacology, pharmacognosy research, and natural product science.