Contribution participating in the INPST 2018 Science Communication Awards contest.
Envision the following possible future clinical scenario: a patient in a hospital develops an antibiotic-resistant bacterial infection that is untreatable or only treatable with the most toxic of antibiotics. During the 48 hours it takes to identify the bacterial species and strain, physicians and scientists also screen a library of bacterium-killing viruses at hand, select those that are effective against this antibiotic-resistant bacterial strain and mix a personalized cocktail of phages to successfully treat the patient.
The first part of the previous hypothetical scenario is already a growing health problem: bacteria that have become resistant to antibiotics and cause deadly diseases in people.
“We are running out of available options to treat patients who have deadly bacterial infections; we need new ideas,” said microbiologist Dr. Anthony Maresso.

One idea is coming from the laboratories of Maresso and his colleagues at Baylor College of Medicine and the Michael E. DeBakey Veterans AffairsMedical Center. They propose that forming an alliance with viruses that kill bacteria, called bacteriophages, might one day help fight back antibiotic-resistant infections.
Although this alliance is still in the future, the researchers’ work in the lab is showing that it is worth exploring in greater detail.
How deadly infections happen
When bacteria grow out of control, they can enter the blood stream and infect vital organs in the body. The body’s immune system, an army of cells and molecules that fights back infections and other diseases, responds to the bacterial attack, defending the body from the infection. However, the immune response sometimes is excessive and can lead to tissue damage, organ failure and death, a process called sepsis. To end sepsis, bacterial growth has to stop. Antibiotic treatment usually can control bacterial growth and prevent the deadly consequences of sepsis, but an increasing number of bacteria is becoming resistant to antibiotics.
According to the National Institute of General Medical Sciences, sepsis affects more than 1 million people in the United States every year. About half of the patients with sepsis die; this outnumbers the U.S. deaths caused by prostate cancer, breast cancer and AIDS combined. The number of sepsis cases per year is increasing, which underscores the need for new strategies to fight bacterial infections.
Maresso and colleagues investigated the possibility of recruiting phages in the fight against antibiotic-resistant bacteria, reviving the original idea of Felix d’Herelle, proposed in 1926.
“The driving force behind this project was to find phages that would kill 12 strains of antibiotic-resistant bacteria that were isolated from patients,” said Dr. Robert Ramig. “As the virologist on the team, my first contribution was to go phage hunting.”

Phage hunting
“I have a number of phages in my lab, but none of them killed the antibiotic-resistant E. coli we were working on – the sequence type 131 currently pandemic across the globe,” Ramig said.

Birds and dogs often carry the bacteria the researchers were interested in, and may be one environmental reservoir of these pathogens. They also carry phages specific for those bacteria. Ramig, Maresso and Sabrina Green, a graduate student in the Maresso lab, went phage hunting in local parks and bird refuges, collecting avian and canine feces.
“We isolated a number of phages from animal feces,” said Ramig. “No single phage would kill all the 12 bacterial strains, but collectively two or three of those phages would be able to kill all of those bacteria in cultures in the lab.”
Encouraged by this good news, the researchers moved on to the next step – determining whether the phages also would be able to kill the antibiotic-resistant bacteria in an animal model of sepsis.
A mouse model of human sepsis
One of the animal models the researchers worked with mimics how cancer patients develop potentially life-threatening infections during their cancer treatment.
“A number of cancer patients who undergo chemotherapy sometimes develop infections that come from bacteria that normally live in their own gut, usually without causing any symptoms,” Green said. “Chemotherapy is intended to kill cancer cells, but one of the side effects is that it suppresses the immune system. A suppressed immune system is a major risk factor for infections with these bacteria, which sometimes also are multi-drug resistant.”
Working in Maresso’s lab, Green developed a mouse model in which healthy mice received antibiotic-resistant bacteria that colonized their intestinal tract. These mice showed no sign of disease.
“But when the mice received chemotherapy,” Green said, “the bacteria moved from their intestine to major organs – this led to a fatal sepsis-like infection.”
In this animal model in which the immune system could not keep in check antibiotic-resistant bacteria, Green tested whether the phages would be able to do so.
“When the phages are delivered into the animals, their efficacy in reducing the levels of bacteria and improving health is dramatic,” Maresso said. “But that is not what is truly remarkable,” he continued. “What is remarkable is that these ‘drugs’ were discovered, isolated, identified and tested in a matter of weeks, and for less money than most of us probably spend in a month on groceries.”
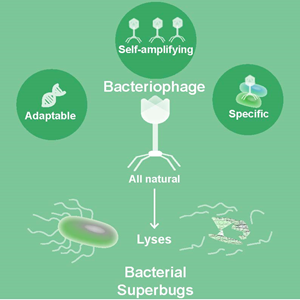
Phages: an adaptable, specific drug
Phages are very specific for certain species or strains of bacteria, but can be made broadly acting via cocktails, if desired. Thus, unlike antibiotics, using phages may not be associated with some of the side effects observed, such as clearing beneficial intestinal microbiota. They also don’t infect human cells.
Another advantage over antibiotics is that phages can evolve. Should resistance develop against one set of phages, new phages can be identified in the environment or evolved in the laboratory in a matter days. “On the other hand, an antibiotic is a chemical; it cannot change in real time,” Maresso said. “It may take years to develop a new antibiotic and at costs that can run in the billions. But a phage can evolve to efficiently kill a resistant strain and then be propagated. It gives me great personal satisfaction when I think of the irony of this – the next anti-bacterial treatment may use the very same mechanisms bacteria have been using against us for 60-plus years now.”

Another member of the research team, Dr. Barbara Trautner, and Ramig had previously published a paper in which they showed that it is possible to take advantage of the phages’ ability to change to fight bacterial infections. “In summary, we took four phages that specifically attacked bacteria of the group Pseudomonas, and they would kill four of 26 of these bacterial strains. Then, we evolved the phages in the lab, and in a month the new ones could kill 22 of the 26,” Ramig said.
“There are many ways to kill bacteria, but I know of no other way that has the potential to evolve in real time like phages do. And it’s the best ‘green’ medicine – it’s natural, safe thus far, relatively cheap and can be harnessed with the technical skills of a college biology major,” Maresso said.
Whereas the upside maybe high, there is still caution. “Phages are not infallible medicines,”reflects Maresso. “The host’s immune system sometimes can neutralize their activity and some phages just don’t work well in animals. But we understand very little about any of these dynamics compared to those of other classes of drugs. At the very least, I think the evidence supports the notion that we should be giving phages experimental attention.”
Read all the details of this research in Scientific Reports.
Dr. Anthony Maresso is an associate professor of virology and microbiology at Baylor College of Medicine.
Dr. Robert Ramig, professor of virology and microbiology at Baylor.
Sabrina Green, a graduate student in the Molecular Virology Program at Baylor.
Dr. Barbara Trautner, is an associate professor and director of clinical research in the Department of Surgery and an associate professor of medicine at Baylor and the Michael E. DeBakey VA.
JasonT. Kaelber, predoctoral fellow of biochemistry at Baylor, also contributed to this project.
This work was supported by a grant from the Mike Hogg Foundation and seed funds from Baylor College of Medicine. Cryoelectron microscopy was performed at the National Center for Macromolecular Imaging at Baylor and supported by grant P41GM103832.
This blog post was previously published at: https://bit.ly/2EmWvjK
Keywords: phages, sepsis, bacteriophages, antibiotics, multidrug resistant (MDR) bacteria, antimicrobial resistance (AMR), antibiotic-resistant bacterial infection.
Join for free INPST as a member
The International Natural Product Sciences Taskforce (INPST) maintains up-to-date lists with conferences, grants and funding opportunities, jobs and open positions, and journal special issues with relevance for the area of phytochemistry and food chemistry, pharmacology, pharmacognosy research, and natural product science.