Yang Yang1, Dongdong Wang1, 2, 3*
Contribution participating in the INPST 2018 Science Communication Awards contest.
Bilirubin, a major pigment in bile with specific antioxidant properties, is a standard serum biomarker of liver function, which has particular toxicity due to irreversible damage to the brain and nervous system. Inexplicably, it is inversely correlated with cardiovascular disease risk. Our group found that unconjugated bilirubin (UCB) decreases macrophage cholesterol efflux and reduces the expression of ATP binding cassette A1 (ABCA1). These novel data underscore the complex bioactivity of bilirubin in the context of cardiovascular diseases (CVD) and may encourage further exploration of bilirubin’s effect on cholesterol metabolism and transport.
As a product during heme catabolism, the bile pigment bilirubin was considered a non-functional waste product in the past. However, recent evidence showed the antioxidant effect of mildly elevated serum bilirubin levels, which suggest that bilirubin is a potent physiological antioxidant and may play a potential impact on protecting CVD, tumor development and inflammation.1,2 Atherosclerosis (AS) is considered the most common CVD in the world, one of the critical causes is abnormal cholesterol metabolism. It is reported that many bioactive molecules prevent AS by promoting macrophage cholesterol efflux through the ABCA1 channel protein, which could slow down the pathogenesis of CVD.3,4 Accordingly, is bilirubin involved in the regulation of cholesterol efflux in macrophages and does it play a role in the formation of AS? Our team conducted a series of studies in this direction.
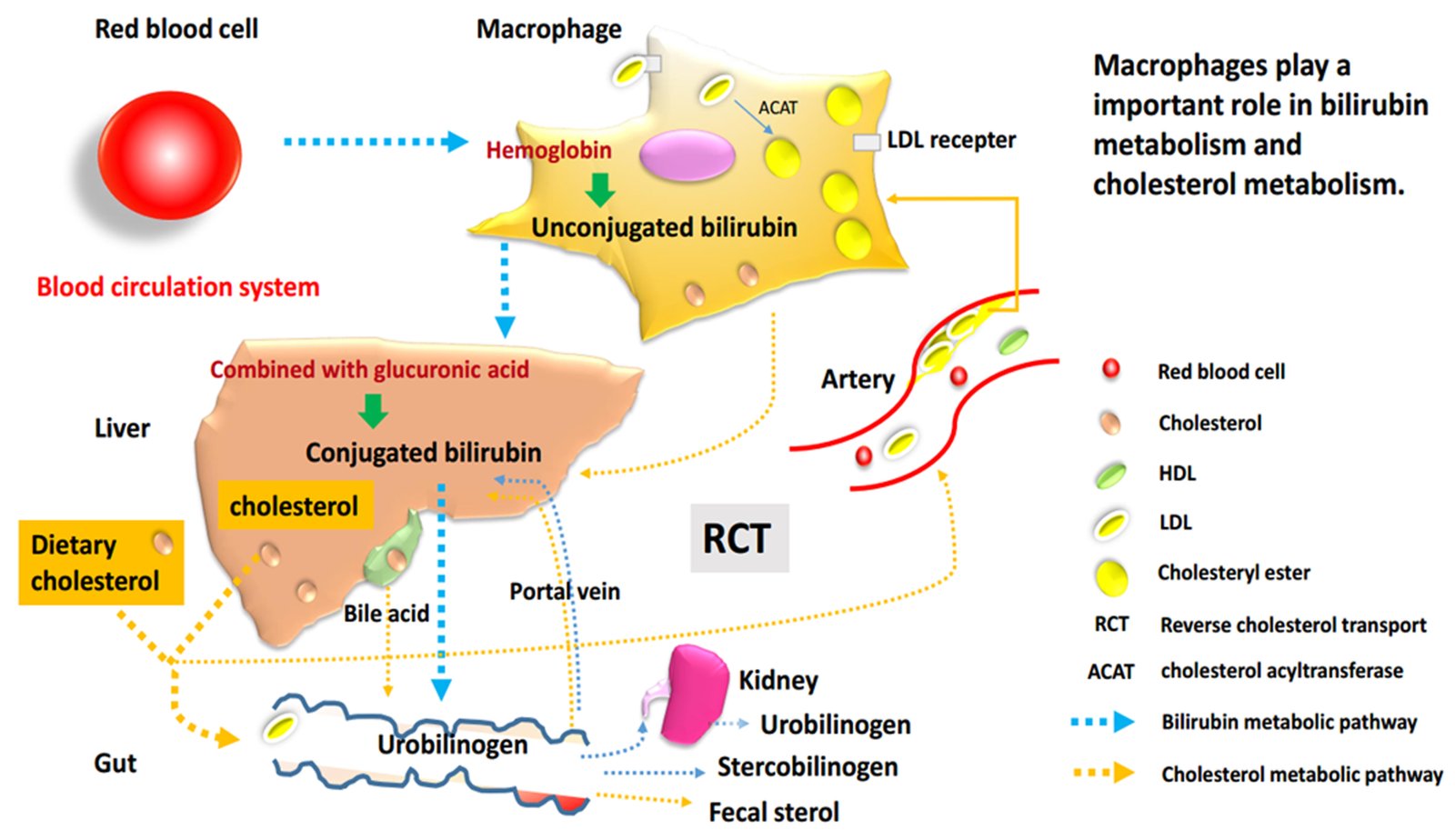
Macrophages, essential cells for bilirubin metabolism and reverse transport of cholesterol
In general, bilirubin is classified into conjugated bilirubin (known as direct bilirubin) and unconjugated bilirubin (UCB, which is also called indirect bilirubin). The mononuclear macrophage system in liver, spleen and bone marrow always phagocytizes aging and abnormal red blood cells, decomposes hemoglobin, then produces and releases free bilirubin, which is the early form of UCB. Therefore, macrophages play an essential part in the initial heme metabolism.
According to published research results, bilirubin activates heme oxygenase as well as possesses antiatherogenic effects, which performed the cardioprotective activity associated with oxidative stress through its antioxidant capacity5-9 in protecting against AS, inflammation7,8 and immune cell proliferation.5 Moreover, it has an inhibitory effect on vascular smooth muscle cell proliferation and migration,10-12 and endothelial dysfunction,13,14 which also potentially related to the anti-AS mechanisms. In the same line, it is worth noting that many studies indicated that UCB is inversely associated with the incidence of myocardial infarction, peripheral artery disease, and other CVD,5 especially Gilbert’s syndrome was extensively studied. Gilbert’s syndrome (GS) is a non-hemolytic, unconjugated hyperbilirubinemia characterized by enhanced levels of UCB, reduced thiols, and glutathione.15,16 Large epidemiological studies show that GS patients, who have higher levels of serum UCB appear to be of advantage because of the reduced incidence of cardiovascular events and cancer.17,18 Meanwhile, it is reported that high-normal (10 – 17.1 μmol/L)19 or mildly elevated (17.1–90 μmol/L)20 serum bilirubin is negatively associated with the risk of developing CVD, which might be related to inhibition of free radical lipid and low density lipoprotein (LDL) oxidation.21,22 But interestingly such a study observed a U-shaped relationship between circulating bilirubin concentrations and cardiovascular risk. It is reported that both the patients who have higher bilirubin levels (12–86 µmol/L) and lowest bilirubin levels (<7 µmol/L) all have the risk of ischemic heart disease.23
As it is widely known, the formation of macrophage-derived foam cells is a typical feature of AS, which is closely related to the abnormal lipid metabolism in cells. On the one hand, excessive intake of cholesterol or reduction/block of efflux can cause the accumulation of lipids and convert macrophages into foam cells. On the other side, cholesterol efflux from macrophages is the first step in the reverse cholesterol transport (RCT), and it is also a critical process in RCT.24 In general, there are many proteins involved in this process, especially ABCA1, ATP binding cassette transporter G1 (ABCG1) and scavenger receptor class B type I (SR-BI). ABCA1 is confirmed as a significant mediator of cholesterol efflux to lipid‐poor apolipoprotein A1 (often referred to as pre‐β HDL), which promotes cholesterol metabolism and acts as an anti-AS role. Besides, some studies have shown that ABCA1 knockout mice phenotype accelerated the pathological progression of AS due to a decrease in macrophage cholesterol efflux to apolipoprotein A1 (apoA1) and high-density lipoprotein (HDL).25,26 It was also confirmed that increased macrophage cholesterol efflux could help to prevent or treat AS.27,28
From the above analysis, it can be seen that macrophages could occupy an essential place in both heme metabolism and cholesterol metabolism pathways. Whether in the production of UCB or the process of RCT, this cell type is of crucial importance. Moreover, all the above research results suggest that serum bilirubin is also significantly and inversely associated with CVD as well as is the macrophage cholesterol metabolism. Therefore, we focused on study on how bilirubin affects the development and progression of CVD through interaction with the lipid metabolism pathway.
Role of bilirubin in macrophage cholesterol efflux
How bilirubin affects atherosclerosis by regulating lipid metabolism? For many years, studies have shown that higher concentrations of UCB are toxic to humans, but more and more scientists reported that plasma bilirubin is correlated with LDL-cholesterol (LDL-C) and HDL-cholesterol (HDL-C) 29,30, which may affect the formation and development of foam cells in AS. Therefore, we examined the effects of bilirubin on macrophage cholesterol efflux and potential associated molecular mechanisms.
At first, we collected blood samples from 60 GS patients and 60 healthy control people, as well as 20 hyperbilirubinemic Gunn rats (a congenital unconjugated nonhemolytic hyperbilirubinemia animal model) and 20 control rats, to compare and research the level of cholesterol efflux in plasma. After statistical analysis of the experimental results, it is observed that there was a reduction (7%) in cholesterol efflux ability mediated by hyperbilirubinemic plasma obtained from GS patients, and a similar reduction (27%) in the Gunn rat group. These data indicated that mild and chronically elevated UCB could induce a significant drop in cholesterol efflux compared with control groups.
Secondly, a series of interesting cell experiments were designed to explore the possible mechanism of bilirubin in macrophage cholesterol efflux. The THP-1 (human monocytic ) cell line was used in these experiments. By adding bilirubin to normobilirubinemic plasma, cholesterol efflux was inhibited in THP-1 macrophages. Even after the removal of bilirubin, the cholesterol efflux ability of THP-1 cells, which had been exposed to bilirubin, was still decreased. In other words, more cholesterol could not be transported out of macrophages, which may indirectly promote the formation of macrophage foaming.
Then, through extensive cell and molecular experiments, our group discovered that bilirubin inhibits cholesterol efflux from THP‐1 macrophages in the presence of human plasma or apoA1 as acceptor. At the same time, it was confirmed that plasma-/apoA1–mediated cholesterol efflux could also be inhibited by UCB concentration- and time-dependently. In detail, we chose different concentrations of bilirubin (3, 10, and 17.1 µmol/L) and time points (4, 8, 16, and 24 hours) to test the level of cholesterol efflux. The results showed that >90% apoA1–mediated cholesterol efflux can be inhibited at 16 and 24 hours. Moreover, low concentrations of UCB do not affect cell viability, only high concentrations (UCB=17.1 µmol/L) have an adverse impact. Moreover, Western blots indicated that UCB could decrease ABCA1 protein expression in THP-1 macrophages and peripheral blood mononuclear cells (PBMCs) from GS patients. Notably, the degradation rate of ABCA1 protein is also accelerated by bilirubin in THP-1. These results suggest that the next study can focus on the ABCA1 related signal pathway. Finally, considering the U-shaped relationship between circulating bilirubin concentrations and CVD risk, there must be more relevant mechanisms worth exploring in the future.
In conclusion, we have demonstrated that the addition of a given concentration of UCB (3-17.1 μmol/L) to plasma/apoA1 supplemented medium can reduce macrophage cholesterol efflux in a concentration- and time-dependent manner. These data improve our knowledge concerning bilirubin’s impact on cholesterol transport and represent a significant advancement in our understanding of the role of bilirubin in CVD.
References:
- Fevery J. Bilirubin in clinical practice: a review. Liver Int. 2008 May;28(5):592-605. doi: 10.1111/j.1478-3231.2008.01716.x.
- Vítek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15(25):2869-83.
- Wang D, Hiebl V, Ladurner A, et al. 6-Dihydroparadol, a Ginger Constituent, Enhances Cholesterol Efflux from THP-1-derived Macrophages. Mol Nutr Food Res. 2018 May 26:e1800011.
- Lu Y, Jia YP. Quercetin upregulates ABCA1 expression through liver X receptor alpha signaling pathway in THP-1 macrophages. Eur Rev Med Pharmacol Sci. 2016 Sep; 20(18):3945-3952.
- Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 2000;46:1723–1727.
- Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996;51:859–862.
- Stocker R, Yamamoto Y, McDonagh A, Glazer A, Ames B. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046.
- Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849.
- Bakrania B, Du Toit EF, Wagner KH, Headrick JP, Bulmer AC. Pre- or postischemic bilirubin ditaurate treatment reduces oxidative tissue damage and improves cardiac function. Int J Cardiol. 2016;202:27–33.
- Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, GracaSouza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039.
- Ollinger R, Yamashita K, Bilban M, Erat A, Kogler P, Thomas M, Csizmadia E, Usheva A, Margreiter R, Bach FH. Bilirubin and biliverdin treatment of atherosclerotic diseases. Cell Cycle. 2007;6:39–43.
- Peyton KJ, Shebib AR, Azam MA, Liu XM, Tulis DA, Durante W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and Migration. Front Pharmacol. 2012;3:48.
- Rodella L, Lamon BD, Rezzani R, Sangras B, Goodman AI, Falck JR, Abraham NG. Carbon monoxide and biliverdin prevent endothelial cell sloughing in rats with type I diabetes. Free Radic Biol Med. 2006;40:2198–2205.
- Yoshino S, Hamasaki S, Ishida S, Kataoka T, Yoshikawa A, Oketani N, Saihara K, Okui H, Shinsato T, Ichiki H, Kubozono T, Kuwahata S, Fujita S, Kanda D, Nakazaki M, Miyata M, Tei C. Relationship between bilirubin concentration, coronary endothelial function, and inflammatory stress in overweight patients. J Atheroscler Thromb. 2011;18:403–412.
- Whitmer DI, Gollan JL. Mechanisms and significance of fasting and dietary hyperbilirubinemia. Semin Liver Dis 1983; 3: 42–51.
- Kundur AR, Singh I, Bulmer AC. Bilirubin, platelet activation and heart disease: a missing link to cardiovascular protection in Gilbert’s syndrome? Atherosclerosis. 2015 Mar;239(1):73-84. doi: 10.1016/j.atherosclerosis.2014.12.042. Epub 2014 Dec 24.
- C. Bulmer, K. Ried, J.T. Blanchfield, K.H. Wagner. The anti-mutagenic properties of bile pigments. Mutat. Res., 658 (1–2) (2008), pp. 28-41
- Ohnaka, S. Kono. Bilirubin, cardiovascular diseases and cancer: epidemiological perspectives. Expert Rev. Endocrinol. Metab., 5 (6) (2010), pp. 891-904
- Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18–23.
- Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GNJ, Jansen PLM, Elferink RPJO, Chowdhury NR. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–1175.
- Novotný, L. Vítek. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp. Biol. Med., 228 (5) (2003), pp. 568-571
- Tsimikas, P. Willeit, J. Willeit, P. Santer, M. Mayr, Q. Xu, A. Mayr, J.L. Witztum, S. Kiechl. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol., 60 (21) (2012), pp. 2218-2229.
- Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. 1995;41:1504–1508
- Phillips, M. C. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 2014, 289, 24020-24029.
- Yvan Charvet L, Ranalletta M, Wang N, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest, 2007, 117 (12): 3900.
- Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATP binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice[J]. Circ Res, 2013,112 (11): 1456.
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135.
- Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393.
- Schwertner HA. Association of smoking and low serum bilirubin antioxidant concentrations. Atherosclerosis 1998;136:383-387
- Madhavan M, Wattigney WA, Srinivasan SR, Berenson GS. Serum bilirubin distribution and its relation to cardiovascular risk in children and young adults. Atherosclerosis 1997;131:107-113.
*The authors are affiliated with:
1) Institute of Genetics and Animal Breeding of the Polish Academy of Sciences, ul. Postepu 36A, 05-552, Jastrzebiec, Poland.
2) Department of Pharmacognosy, University of Vienna, Althanstrasse 14, 1090, Vienna, Austria.
3) Institute of Clinical Chemistry, University Hospital Zurich, University of Zurich, Wagistrasse 14, 8952, Schlieren, Switzerland.
Keywords: cardiovascular disease risk, bilirubin, macrophage cholesterol efflux, ATP binding cassette A1, ABCA1, CVD, heme catabolism, atherosclerosis, unconjugated bilirubin, antiatherogenic effects, cardioprotective activity, Gilbert’s syndrome, reverse cholesterol transport, RCT, HDL, LDL, hyperbilirubinemic Gunn rats, apoA1 .
Join for free INPST as a member
The International Natural Product Sciences Taskforce (INPST) maintains up-to-date lists with conferences, grants and funding opportunities, jobs and open positions, and journal special issues with relevance for the area of phytochemistry and food chemistry, pharmacology, pharmacognosy research, and natural product science.
Leave a comment: